Anal and Peri-Anal Warts
What are Anal Warts?
Anal warts are one of the most common sexually transmitted infections in world. They are caused by a small DNA virus, a papillomavirus belonging to the papovavirus group, which cannot be cultured. Genital warts differ from skin warts histologically and antigenically. Genital warts are nearly always
transmitted by sexual contact; autoinoculation from hand to genitals is unusual. The infectivity of sexually acquired warts is about 60%; the incubation period is long, varying from two weeks to eight months.
Four distinct sub-types of Ano-genital warts have been described: condylomata acuminata (pointed warts), flat / macular lesions, papular, and keratotic lesions. The first two sub-types are mainly found on moist, non-keratinized epithelia, while the latter two usually present on keratinized epidermis. Ano-genital warts are also often referred to as genital warts, condylomata acuminata or genital verruca.
Anal/ peri-anal/ Ano-genital warts are highly infectious; approximately 65% of individuals with an infected partner develop the lesions within 3 weeks and 8 months after exposure, the median time between infection with HPV types 6 or 11 and the development of warts was 11 to 12 months among males and 5 to 6 months among young females. In rare cases, Ano-genital warts can be associated with malignant lesions, namely Buschke-Lowenstein tumors.
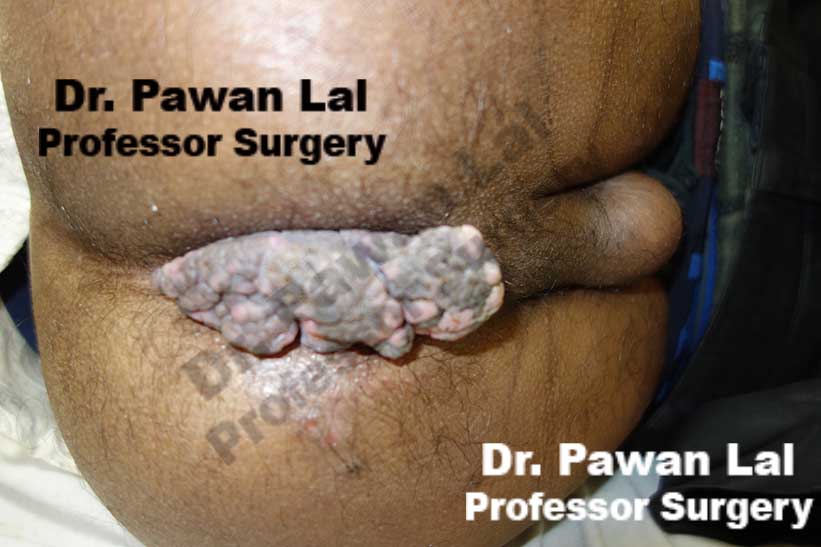
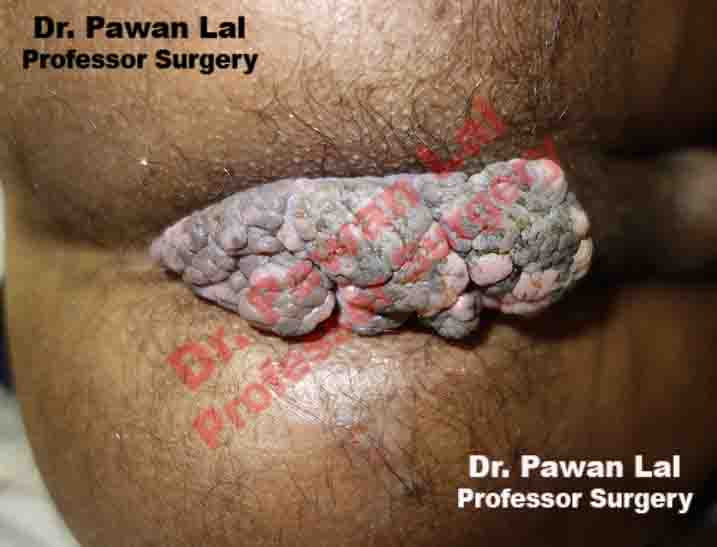
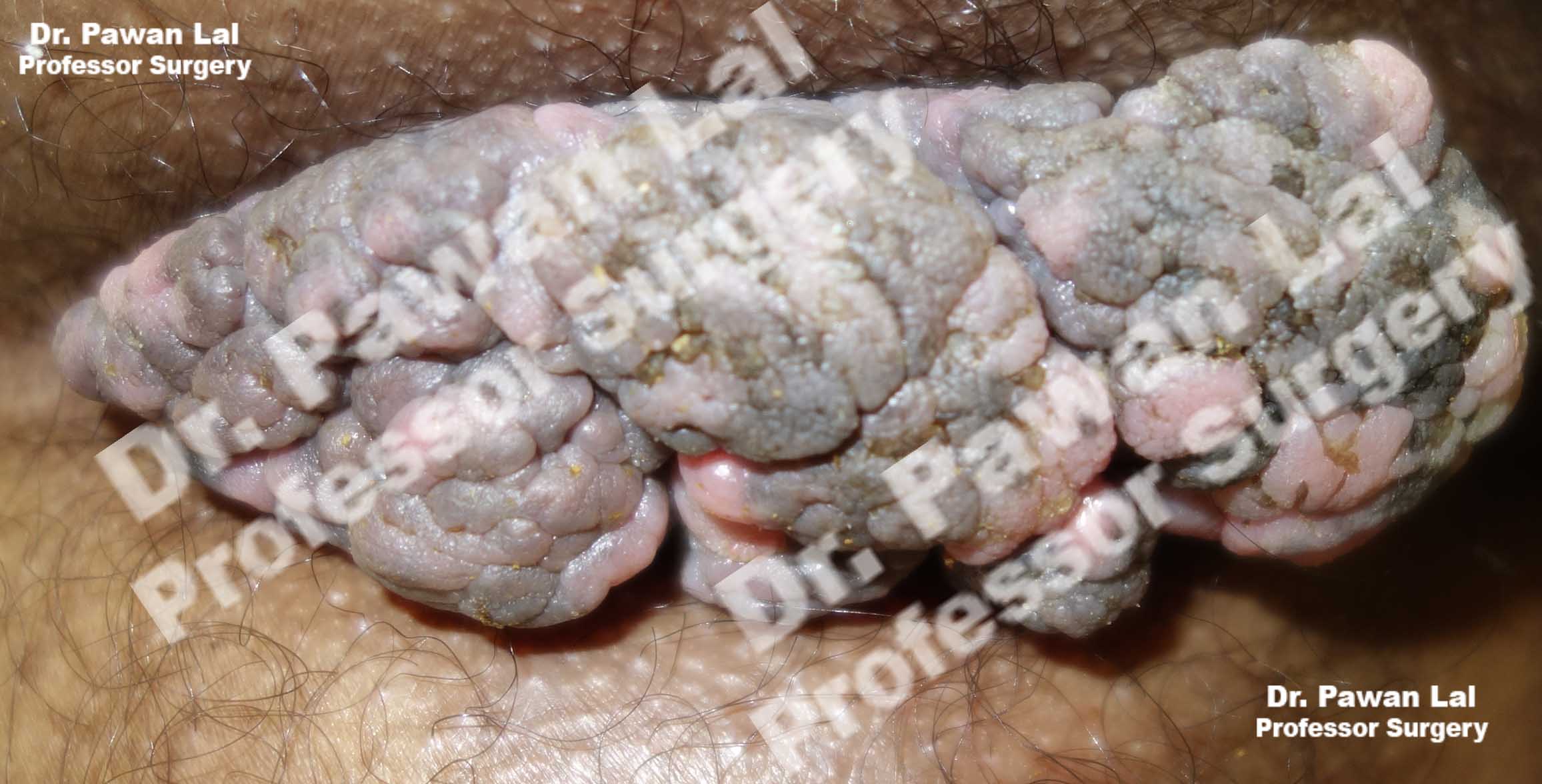
 Multiple Peri-anal warts (Condyloma acuminata)
Multiple Peri-anal warts (Condyloma acuminata)
Multiple Peri-anal warts (Condyloma acuminata)
Diagrammatic representation of Anal Warts is shown below
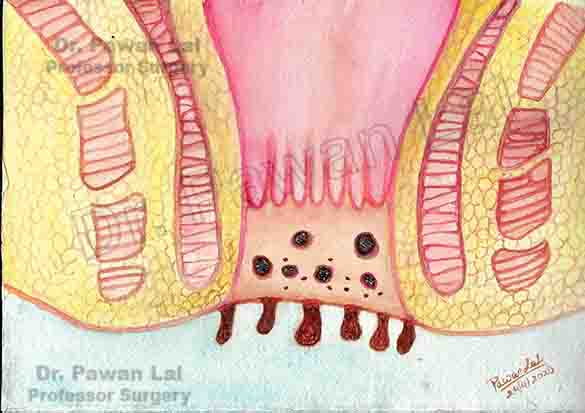
Diagram showing warts in the Anal canal and peri-anal skin (Also Anal verge)
Peri-anal and Anal Warts (Diagramatic)
Peri-anal and Anal Warts (Diagramatic)
Symptoms of Anal Warts
Anal warts are a commonly transmitted sexual infection among young adults. It is a painless condition and most are unaware as there are no symptoms. There is a common complain of anal itching. Tiny spots or growths can be seen and also can be felt with fingers. When they become large, one may feel a lump or mass in the anal area. The other symptoms noticed in anal or peri-anal warts are bleeding or increased mucus discharge. pain, itching, burning, irritation,
The infection with Human papilloma virus (HPV) can cause emotional and psychological problems with the person which includes shame, embarrassment, anger, depression and guilt. Since the Anal and Peri-anal warts are caused by ano-receptive sex, they are either not reported early either due to non-visualization or due to shame/guilt. The lesions can be handled easily when they are reported early. Presence of peri-anal lesions can cause cessation of sexual activity of patients, either through fear of transmission or embarrassment of lesions.
Causes of Anal warts
Cutaneous (skin) HPV types
Most HPV types are called cutaneous because they cause warts on the skin, such as on the arms, chest, hands, and feet. These are common warts, not genital warts.
Mucosal (Genital or Anogenital) HPV types
The other HPV types are considered mucosal types because they invade and live in cells on mucosal surfaces. The mucosal HPV types are also called genital (or anogenital) HPV types because they often affect the anal and genital area. These types can also infect the lining of the mouth and throat. Mucosal HPV types generally don’t grow in the skin or parts of the body other than the mucosal surfaces but can involve anal and perianal skin surface as shown in the picture below. Mucosal (genital) HPV is spread mainly by direct skin-to-skin contact during vaginal, oral, or anal sexual activity. It’s not spread through blood or body fluids. It can be spread even when an infected person has no visible signs or symptoms.
Click for large image
Low-risk mucosal (genital) HPV types: HPV types that tend to cause warts and rarely cause cancer are called low-risk types. Low-risk genital HPV infection can cause cauliflower-shaped warts on or around the genitals and anus of both men and women. In women, warts may appear in areas that aren’t always noticed, such as the cervix and vagina.
High-risk mucosal (genital) HPV types: HPV types that can cause cancer are called high-risk types. These types have been linked to certain cancers in both men and women. Doctors worry about the cell changes and pre-cancers these types cause because they are more likely to grow into cancers over time6.
Anyone who has had sexual contact can get HPV, even if it was only with only one person, but infections are more likely in people who have had many sex partners.
The virus can also be spread by genital contact without sex, but this is not common. Oral-genital and hand-genital spread of some genital HPV types have been reported. And there may be other ways to become infected with HPV that aren’t yet clear.
You DO NOT get HPV from:
- Toilet seats
- Hugging or holding hands
- Swimming in pools or hot tubs
- Sharing food or utensils
- Being unclean
Transmission from mother to newborn during birth is rare, but it can happen, too. When it does, it can cause warts (papillomas) in the infant’s breathing tubes (trachea and bronchi) and lungs, which is called respiratory papillomatosis. These papillomas can also grow in the voice box, which is called laryngeal papillomatosis. Both of these infections can cause life-long problems.
Risk Factors of Anal Warts
- Multiple sexual partners
- Anal receptive intercourse
- Not using barrier contraceptives, such as condoms
Prevention of Anal Warts
Human papillomavirus vaccine is available that prevents the HPV infection and formation of anal warts.
Other preventive measures include:
- Using condoms
- Limiting sexual contact to a single partner
- Sexual abstinence
Condoms can offer some protection from HPV infection, but HPV might be on skin that’s not covered by the condom. And condoms must be used every time, from start to finish. The virus can spread during direct skin-to-skin contact before the condom is put on, and male condoms don’t protect the entire genital area, especially for women. The female condom covers more of the vulva in women, but hasn’t been studied as carefully for its ability to protect against HPV. Condoms are very helpful, though, in protecting against other infections that can be spread through sexual activity.
It’s usually not possible to know who has a mucosal HPV infection, and HPV is so common that even using these measures doesn’t guarantee that a person won’t get infected, but they can help lower the risk 10.
Anogenital warts can now be effectively prevented using the quadrivalent (HPV 6, 11, 16 and 18) or nanovalent (HPV 6, 11, 16, 18, 31, 33, 45, 52 and 58) HPV vaccines; these protect against HPV types that cause anal/peri-anal/anogenital warts, cervical cancer and other types of anogenital and oral cancer. The Human Papilloma Virus (HPV) quadrivalent vaccine has shown to be up to 100% effective in preventing AGW in association with vaccine‐type HPV in women. The vaccine is also effective in 12‐ to 15‐year‐old boys and is licensed for use in both sexes. Evidence on whether the vaccination could be useful in anogenital warts treatment is not yet clear; however, there are scientific data supporting use of the vaccination in individuals previously exposed to HPV.
Click here for more details about HPV Vaccine
Complications of Anal Warts
Anal warts are not life-threatening. If untreated, anal warts increase in size and can cause bleeding and discomfort. The risk of anal cancer also increases with some HPV subtypes 16 and 18. The risk of anal cancer is about 17 times higher in sexually active gay and bisexual men than in men who have sex only with women. Men who have HIV (human immunodeficiency virus) are also at higher risk of getting this cancer. Most cancers that are found in the back of the throat, including at the base of the tongue and in the tonsils, are HPV related due to oral sex.
Oral HPV infections - those persons doing oral sex like sucking and licking the sexual organs (cunnilingus/ fellatio/ anilingus/ rimming) can develop oral HPV infections involving the oral cavity, throat, palate, tonsils and tongue. These HPV infections over long time can cause oral and throat cancers.
Diagnosis of Anal Warts
A detailed history regarding the sexual practices, anal receptive intercourse, and previous history of sexual transmitted diseases will be taken. Warts are diagnostic on appearance. Anal / peri-anal warts appear as papillomatous plaques or flat lesions and can be single or multiple in number. Lesions vary from flesh‐coloured to white, pink or brown. They typically manifest in areas of the body that are in close contact during sex: mainly on the anogenital areas such as vulva, penis, groin, perineum, perianal skin, Anal canal but also in the oral cavity (in case of oral sex). Diagnosis of clinically typical and does not require histological confirmation. DRE - Digital rectal examination is a must in Anal and Peri-anal warts to rule out intra-anal and rectal warts.
Patients should be reassured that if they have developed anal/ peri-anal / Ano-genital warts, appropriate treatment can clear the warts within 3 months. The anal/peri-anal lesions are of mostly sexual origin and are caused by Human Papilloma Virus (HPV) which is contagious; therefore, it is important for patients to disclose their recent sexual partners, who should be advised examination to rule out Human papilloma virus (HPV) infection. The smokers have a 27% increased risk of developing anal/peri-anal (ano-genital) warts as compared with non‐smokers. The HPV prevalence in patients who smoke is 48.2% compared with 37.5% for non‐smokers (P < 0.001). Generally, warts develop within weeks or months after acquiring Human Papilloma Virus (HPV) infection but in a significant number of cases, the virus can be dormant for months or years before warts emerge.
HPV Screening Through Anal Cytology
Though there is no guidelines for anal screening for HPV or anal cytology for anal cancer lesion (precancerous or abnormal anal canal cytology) but there is benefit if getting the anal Cytology for Anal cancer screening (similar to cervical cancer screening), using anal cytology (through canal brush cytology) followed by referral of patients with abnormal results to high-resolution anoscopy and subsequent treatment of biopsy-proved AIN (Anal canal Cancer in situ), may prevent the development of anal cancer.
The reported sensitivity and specificity of anal cytology relative to findings at biopsy (sensitivity, 69%-93%; specificity, 32%-59%, respectively) are similar to findings in studies comparing cervical cytology and cervical biopsy for the prevention of cervical cancer. There is definitive benefit to do annual anal cytology for MSM (men having sex with men) and any HIV-positive patients with a history of anogenital condylomas. Among patients with HIV- or HPV-related lesions, histologic signs of dysplasia are apparent in more than one-fifth of those who undergo testing. Among HIV-positive MSM (men having sex with men), the positive predictive value of abnormal anal cytology to predict anal dysplasia is approximately 95%.
Anal cytology can distinguish between the various stages of Anal Intra-epithelial neoplasia (AIN) 9.
- Low-grade dysplastic cellular changes (AIN 1).
- severe dysplasia (AIN 2)
- Severe dysplasia and cancerous changes but no invasion (AIN 3), also known as carcinoma in situ, is diagnosed when 50% of the epithelial tissue is replaced with abnormal dysplastic cells.
Buschke–Lowenstein Tumor - Buschke–Lowenstein tumor, often called giant condyloma accuminatum, is considered by some authors as intermediate between condyloma and squamous cell carcinoma. Histologically, the tumor appears benign with papillomatosis, epithelial hyperplasia, and koilocytosis, but clinically it can behave aggressively with extensive infiltration. Typically, it is slow growing in immunocompetent individuals, but it can grow rapidly in immunocompromised individuals. Focally, these tumors can transform into invasive carcinoma; hence, early diagnosis and treatment is crucial. Common treatment approach includes radical surgical resection with tumor-free resection margins. Prophylactic HPV vaccination has been shown to reduce HPV6/11 infection and anogenital condylomata and thus is expected to prevent this tumor12, 13, 14.
Technique of anal Cytology for collecting the specimen
The goal of anal cytology is to identify patients with cellular changes in the epithelial cells that line the anal canal; any patients with atypia are then referred to undergo high-resolution anoscopy. No specific preparation is necessary before anal cytology, though patients should be instructed to refrain from receptive anal sex and enemas for 24 hours before testing. If a digital rectal examination is performed in conjunction with anal cytology, the cytologic sample must be obtained before lubrication is introduced into the anal canal. The standard technique used in obtaining anal cytologic specimens involves inserting a water-moistened Dacron swab into the anal canal to above the squamocolumnar transition zone, approximately 2 cm (1 inch) from the anal verge. While mild external pressure is applied to the anal wall, the swab is gently manipulated in a craniocaudal and circular motion within the canal. After several rotations, the swab should be withdrawn and immediately immersed in methanol-based preservative-transport solution.
High Resolution Anoscopy by high powered microscope (Anoscope / colposcope) for screening for intra-epithelial tumors of anal canal
The patient is put in jack knife position to expose anal canal. The anal canal is opened by inserting a Anal retractor (Czerny rectal speculum)

The anal canal is then screened segment by segment. The anal skin is treated with acetic acid 3% to see any aceto-white lesions and then treated with Lugol's iodine. Any area that does not take up the iodine is suspcious area and biopsy is taken from that area.
Treatment of Anal Warts
Treatment options include patient-applied (home-based) chemical treatments (podofilox, imiquimod), physician applied (office-based) chemical treatments (podophyllin, trichloracetic acid, interferon, green tea extract) and ablative treatments (cryotherapy, surgical removal, laser treatment). The main limitation of current therapies is the high recurrence rate after initial remission. The quadrivalent HPV vaccine demonstrated high efficacy in preventing the onset of HPV 6/11-related Ano-genital Warts in both males and females.
Treatment options are:
- Topical medicines – putting cream or a liquid on to the warts (trichlorocetic acid, podophyllin, imiquimod).
- Ablative techniques: Freezing (cryotherapy using liquid nitrogen), Electro-cautery (under local anaesthesia), Laser ablation (under local anaesthesia)
- Surgical excision
- Immuno-modulation
Untreated anal warts may grow and increase in size. It is, therefore, better to treat them and remove them. The treatment will depend on the size, number, and location of warts. For small warts. Untreated anal warts may grow and increase in size. It is, therefore, better to treat them and remove them. The treatment will depend on the size, number, and location of warts.
Treatment should be individualized for each patient. Although untreated warts can resolve spontaneously, most patients want an immediate intervention to eradicate them. Treatments need to be selected on the basis of considerations such as the number, size, morphology, location and keratinization of warts, and whether they are new or recurrent.
Ablative techniques
Ablative techniques are commonly used to remove warts. The major frustration is the high rate of recurrence with these treatments and the need for repeat therapeutic interventions. Ablative techniques are associated with a risk of bleeding, tissue destruction, slow wound healing and scarring
| Treatment | Mode of action | Schedule | Clearance rate (%) | Recurrence rate (%) | Advantages | Disadvantages |
| Ablative Techniques | ||||||
| Electrocautery | High‐frequency electrical currents cause thermal damage to infected tissue | Under local anaesthesia, base of lesion excised; repeat as required |
90% (Range:35–94%) |
20% (Range:20-25%) |
90% |
High recurrence rate is used alone but with immunomodulator creams the results are more than 90% remission rate Repeat physician visits Expertise required Smoke evacuator needed |
| CO2 and Nd:YAG laser | Laser vaporizes lesions | Under local anaesthesia, protocol depends on type of laser |
90% (Range:23–95%) |
70% (Range:2.5–77%) |
Rapid results Effective for thick lesions |
High recurrence rate; in some cases even before healing of laser treatment Costly Substantial training & Expertise required Pain/scarring Smoke evacuator needed |
Electrocautery or Cautery
Electrocautery uses high‐frequency electrical currents to destroy anal / peri-anal / anogenital lesions and requires local anaesthesia. Clinical studies have shown clearance rates of 35–94%. As fumes from electrocautery contain contagious particles, preventative measures should be put in place to stop the virus spreading.
Electrofulgration/ electrocautery - will generate smoke/fumes which may contain human papillomavirus (HPV) particles that may be transmitted to the operator who breaths in or comes into contact with the fume15, 16, 17, 18. When working with HPV-related lesions, minimise the risk of transmission by -
-
Use smoke evacuator with intake nozzle 2 cm from operative site
-
Wear surgical mask (N95 is most effective) and eye protection.
Management of Ano-genital warts - part 2
Fulguration of Warts
Click here to watch this video of Fulgration of Warts on youtube
Management of Ano-genital warts - part 4
Fulgration of Intra-anal warts and peri-anal warts is demonstrated
Click here to watch this video of management of Intra-anal warts on youtube
Laser ablation
Carbon dioxide (CO2) and Nd:YAG lasers, Argon Laser or other laser energies vaporize lesions using focused light energy; however, it is not always possible to know the extent of the infected tissue, and therefore, vaporizing large regions around the warts is not always feasible. Local anaesthesia is usually required, especially on extensive and thick lesions as it can penetrate deeply into the lesions. This treatment option is used less frequently than other therapies as it requires specialized and costly equipment, and has an increased risk of serious complications. However, clearance rates of up to 95% have been reported in clinical studies, with a head‐to‐head comparison. It is important to note that fumes from laser treatment contain contagious particles and adequate measures should be taken to prevent the virus from spreading15, 16, 17, 18. Masks and smoke evacuators should therefore be used.
Spread of Human papilloma virus with surgical smoke15, 16, 17, 18
Surgical smoke is the gaseous byproduct generated by surgical procedures and tools, including electrocautery devices, laser ablation, and ultrasonic scalpels, as a result of the thermal decomposition of tissues. There is substantial evidence indicating the presence of numerous chemicals, blood and tissue particles, viruses, bacteria, and even tumor cells in surgical smoke. HPV DNA could be isolated from the smoke plume of papilloma virus infected warts treated with lasers. There are also reported cases of surgeons treating the ano-genital warts developing laryngeal papillomatosis
after administering laser therapy to patients with anogenital condylomas due to HPV infection which was confirmed by in situ DNA hybridization
of the doctor’s laryngeal papilloma tissues which revealed HPV types 6 and 11, similar to those found in the patients. There are also case reports of surgeons / Gynaecologists developing tonsillar cancer / tongue caners which were HPV - 16 positive after managing ano-genital warts for many years.
After surgical fulgration by electro-cautery / laser, HPV DNA can be detected in the nasal samples of the surgeons after inhalation of surgical smoke.
The HPV DNA in the surgical plume is infectious and thus potentially harmful to healthcare professionals. It is recommended that the diligent use of high-filtration masks, in addition to smoke evacuation systems, by surgeons managing ano-genital warts.
Surgical Management of Anal warts
This is the clip showing surgical excision of the
Giant anal Warts (condyloma Acuminata)
Click here to see this video of surgical excision of
Giant Anal warts (condyloma Acuminata) on youtube
Another case of Giant Condyloma Acuminata
Giant anal warts - Surgical Removal with fulgration
Topical Application Therapy
| Treatment | Mode of action | Schedule | Clearance rate (%) | Recurrence rate (%) | Advantages | Disadvantages |
| Topical therapy | ||||||
| Trichloroacetic acid (33–50%) | Acid induces a chemical burn |
Applied directly to lesions; repeat One to three times per week |
95% (Range: 70 - 100%) |
35% (Range: 18-36%) |
Rapid results suitable for small lesions |
High recurrence rate Intense burning sensation |
| Podophyllotoxin 0.5% (alcoholic solution) 0.15% (cream) | Antimitotic agent induces tissue necrosis | Twice‐daily to affected areas for 3 consecutive days per week; discontinue for 4 days; repeat for up to 4 weeks |
90% (Range:45–95%) |
100% (Range:11-100%) |
Easy self‐application |
Very High recurrence rate Complicated regimen Intense application site reactions |
Trichloroacetic acid (TCA; 33–50%)
Physician‐applied acidic treatment causes a chemical burn that destroys the Anal / peri-anal / ano-genital warts. The acid can be administered up to three times per week until the warts have cleared. This process requires a skilled professional to choose the appropriate lesion and duration of application but it is easy to apply and effective treatment. It has clearance rates of 70–100% reported in clinical studies. However, side‐effects such as local discomfort, burning and ulceration are common, hence the need for careful application.
Podophyllotoxin 0.15% cream or 0.5% alcoholic solution
Podophyllotoxin stops division of infected cells causing tissue necrosis. It can be self‐applied by patients twice‐daily for three consecutive days, separated by a 4‐day treatment‐free period and repeated for up to 4 weeks. Patients need to carefully apply the solution to the lesions and avoid contact with healthy skin. Clearance rates from clinical studies range from 45 to 94%, with common side‐effects including pain, itching, burning, erosion and inflammation4.
Immunotherapies
Imiquimod 5% or 3.75%
Imiquimod is an immune response modifier with antiviral activity. This Toll‐like receptor 7 agonist induces the production of cytokines, which enhance the ability of antigen presenting cells to present viral antigens to reactive T lymphocytes. Imiquimod 5% has been approved for the treatment of anal/perianal/anogenital warts worldwide. Imiquimod 5% is self‐applied by the patient three nights per week for up to 16 weeks; if no improvement has occurred after 4–6 weeks, treatment can be applied daily. Imiquimod 5% may be applied for longer durations if there is a good clinical result but complete clearance has not occurred at the end of the initial treatment period. Imiquimod formulations are associated with local skin reactions such as erythema, pruritus, burning, pain and sometimes erosions. These are all signs that the immune system has been activated. Clearance rates from clinical studies range from 35 to 75% with the 5% cream, with higher clearance rates in women than men.
Imiquimod is an immune response modifier that has potent antitumour and antiviral activity. It induces interferon-á, interleukin 1 (IL-1), and tumour necrosis factor á (TNF-á) in peripheral blood, and human keratinocytes exposed to imiquimod show an increase in mRNA for IL-2, IL-6, and IL-8. Wart clearance was associated with tissue production of interferons α, β and γ, and TNF-α 11.
Click here to know more about Imiquad (Imiquimod Cream) - 5% cream or 12.5 gm in 0.25gm cream sachet
Treatment Options
Anogenital warts recurrence is common and frustrating for patients and physicians. Recurrence rates with conventional ablative techniques are relatively high, since these methods only remove the visible wart without affecting the underlying HPV infection. Of currently available treatments, recurrence rates are very low with immunotherapies, imiquimod (6–19%) and sinecatechins (4–12%) as these treatments stimulate the host's immune response to clear the warts.
Studies have shown that a combination of ablative techniques followed by immunotherapy may lead to even lower recurrence rates; ablation provides rapid clearance but has high recurrence rates while immunotherapy has slow clearance rates and a lower risk of recurrence.
Imiquimod 5% applied within 3 weeks after warts ablation (to ensure complete wound healing) was associated with a low rate of wart recurrence.
Pre‐treatment of Ano-genital warts with imiquimod to stimulate an immune reaction followed by surgery is also associated with low recurrence rates.
For other Misc. treatment options for anal warts click here
Follow Up
The anal warts can recur, even without sexual intercourse. HPV can hide in tissues for many months and can again cause new anal warts to grow. Therefore one may require a repeat treatment or surgery in case of recurrence.
Vaccination
Gardasil 9 is the most common vaccine used and is preventive against 9 HPV serotypes.
GARDASIL 9 is a vaccine indicated in females 9 through 45 years of age for the prevention of cervical, vulvar, vaginal, and anal cancers caused by human papillomavirus (HPV) Types 16, 18, 31, 33, 45, 52, and 58; precancerous or dysplastic lesions caused by HPV Types 6, 11, 16, 18, 31, 33, 45, 52, and 58; and genital warts caused by HPV Types 6 and 11.
GARDASIL 9 is indicated in males 9 through 45 years of age for the prevention of anal cancer caused by HPV Types 16, 18, 31, 33, 45, 52, and 58; precancerous or dysplastic lesions caused by HPV Types 6, 11, 16, 18, 31, 33, 45, 52, and 58; and genital warts caused by HPV Types 6 and 11.
This vaccine can prevent most cases of cervical cancer if given before a girl or woman is exposed to the virus. In addition, this vaccine can prevent vaginal and vulvar cancer in women, and can prevent genital warts and anal cancer in women and men.
In theory, vaccinating boys against the types of HPV associated with cervical cancer might also help protect girls from the virus by possibly decreasing transmission. Certain types of HPV have also been linked to cancers in the mouth and throat, so the HPV vaccine likely offers some protection against these cancers, too
Dosage -- O'Mahony C, Gomberg M, Skerlev M, et al. Position statement for the diagnosis and management of anogenital warts. J Eur Acad Dermatol Venereol. 2019;33(6):1006–1019. doi:10.1111/jdv.15570.
- Patel H, Wagner M, Singhal P, Kothari S. Systematic review of the incidence and prevalence of genital warts. BMC Infect Dis. 2013;13:39. Published 2013 Jan 25. doi:10.1186/1471-2334-13-39.
- McGinley KF, Hey W, Sussman DO, Brown GA. Human Papillomavirus Testing in Men. J Am Osteopath Assoc 2011;111(3_suppl_2):S26–S28.
- Nguyen AL, Quint KD, Bouwes Bavinck JN, Erceg A, de Kort WJA, Körver JEM. Real-life treatment of cutaneous warts with cantharidin podophyllin salicylic acid solution. Dermatol Ther. 2019 Nov;32(6):e13143. doi: 10.1111/dth.13143. Epub 2019 Nov 20. PMID: 31664756; PMCID: PMC6916542.
- Hyun JS, Kim GB, Choi BS, Kim MS, Park SG. Giant anal condyloma (giant condyloma acuminatum of anus) after allogeneic bone marrow transplantation associated with human papillomavirus: a case report. J Med Case Rep. 2015 Jan 19;9:9. doi: 10.1186/1752-1947-9-9. PMID: 25597932; PMCID: PMC4334849.
- Bastola S, Halalau A, Kc O, Adhikari A. A Gigantic Anal Mass: Buschke-Löwenstein Tumor in a Patient with Controlled HIV Infection with Fatal Outcome. Case Rep Infect Dis. 2018 Apr 1;2018:7267213. doi: 10.1155/2018/7267213. PMID: 29808133; PMCID: PMC5902119.
- Demirel AH, Ongoren AU, Bingül F, Gulcelik N. Total excision and V-Y plasty technique in the anal area condyloma acuminatum. Indian J Plast Surg. 2008 Jan;41(1):67-9. doi: 10.4103/0970-0358.41115. PMID: 19753205; PMCID: PMC2739560.
- Deshpande DJ, Nayak CS, Mishra SN, Dhurat RS. Verrucous condyloma lata mimicking condyloma acuminata: An unusual presentation. Indian J Sex Transm Dis AIDS. 2009 Jul;30(2):100-2. doi: 10.4103/0253-7184.62766. PMID: 21938129; PMCID: PMC3168050.
- Echenique I, Phillips BR. Anal warts and anal intradermal neoplasia. Clin Colon Rectal Surg. 2011 Mar;24(1):31-8. doi: 10.1055/s-0031-1272821. PMID: 22379403; PMCID: PMC3140331.
- McClean HL, Hillman RJ. Anogenital warts and condom use--a survey of information giving. Genitourin Med. 1997 Jun;73(3):203-6. doi: 10.1136/sti.73.3.203. PMID: 9306902; PMCID: PMC1195823.
- Eggleton S, Tang A. Management of difficult anogenital warts. Sex Transm Infect. 1999 Dec;75(6):449-50. PMID: 10754962.
- Bastola S, Halalau A, Kc O, Adhikari A. A Gigantic Anal Mass: Buschke-Löwenstein Tumor in a Patient with Controlled HIV Infection with Fatal Outcome. Case Rep Infect Dis. 2018 Apr 1;2018:7267213. doi: 10.1155/2018/7267213. PMID: 29808133; PMCID: PMC5902119.
- Yakan S, Cengiz F, Telciler KE, Uz M, Denecli AG. Rectal Involvement of Recurrent Buschke-Lowenstein Tumor Causing Subileus: a Case Report. Gastroenterology Res. 2011 Aug;4(4):177-179. doi: 10.4021/gr316w. Epub 2011 Jul 20. PMID: 27942337; PMCID: PMC5139731.
- Tas S, Arik MK, Ozkul F, Cikman O, Akgun Y. Perianal giant condyloma acuminatum-buschke-löwenstein tumor: a case report. Case Rep Surg. 2012;2012:507374. doi: 10.1155/2012/507374. Epub 2012 Nov 20. PMID: 23213594; PMCID: PMC3508531.
- Zhou Q, Hu X, Zhou J, Zhao M, Zhu X, Zhu X. Human papillomavirus DNA in surgical smoke during cervical loop electrosurgical excision procedures and its impact on the surgeon. Cancer Manag Res. 2019 Apr 29;11:3643-3654. doi: 10.2147/CMAR.S201975. PMID: 31118787; PMCID: PMC6499148.
- Parker J, Clark J (2021) HPV Positive Oropharyngeal Cancer in Two Gynaecologists Exposed to Electrosurgical Smoke Plume. Obstet Gynecol Cases Rev 8:205. doi.org/10.23937/2377-9004/1410205
- Rioux M, Garland A, Webster D, Reardon E. HPV positive tonsillar cancer in two laser surgeons: case reports. J Otolaryngol Head Neck Surg. 2013 Nov 18;42(1):54. doi: 10.1186/1916-0216-42-54. PMID: 24246045; PMCID: PMC3843579.
- Fox-Lewis A, Allum C, Vokes D, et al. Human papillomavirus and surgical smoke: a systematic review Occupational and Environmental Medicine 2020;77:809-817.